1. Introduction
1.1 Research Question
What is the effect of boiling time (0, 2, 5, 10, 15 and 20) minutes on iron leached from the water spinach (ipomoea aquatica) as determined by titrating acidified Potassium Permanganate against water spinach extract.
1.2 Purpose for investigation
In humans, iron is among the most essential minerals; almost all human cells contain iron while it is predominantly found in red blood cells for transporting oxygen. Iron is most notably used for the synthesis of hemoglobin, a protein found in a red blood cell that facilitates the transport of oxygen to cells around the body. Not only that, iron also plays several vital roles in the immune system and helps to treat anemia just to name a few.

Iron deficiency occurs when the body is short of iron for long periods of time, resulting in anemia and other bone-related problems. According to the Asian bank development, 63.5% of Indonesia’s population suffers from iron deficiency anemia. From Figure 1 It can be seen that anemic people have a lower count of red blood cells in their blood, reducing their oxygen carrying capacity and leading to symptoms such as feeling out of breath and dizziness. One of the most iron rich vegetables out there is water spinach, as it is boiled, it gradually loses its iron content as the Iron (II) ions present dissolve into the boiling water and are lost from the spinach, however it is undetermined how much of the Iron (II) ions actually leak into the surrounding water. This led me to ask myself the question whether it is more nutritionally beneficial to eat vegetables raw or cooked. Thus the purpose of this investigation would be to examine how varying boiling times affect the iron content in spinach as determined by the oxidation of iron (II) ions to Iron (III) ions in the Water Spinach extract. By measuring the amount of iron (III) ions present in the Water Spinach extract, we can calculate the amount of iron (II) ions remaining in spinach after boiling.
1.3 Background Information
1.3.1 Water Spinach
Gram for gram, spinach has one of the highest concentrations of iron relative to other vegetables. 100 g of cooked spinach provide 3.5mg of iron which is already 20% of the recommended daily amount. Hence it is an excellent source of nutrition and effective for preventing anemia.
A common species of spinach found in South East Asia is water spinach or kangkong. Water spinach is indigenous to SouthEast Asia and grows best in tropical and humid climates near the equator. Rich in a wide variety of nutrients, particularly iron, it has become a staple source of food and nutrition for those living in the region thanks to its high nutritional value and relatively low price. Like most spinach, water spinach contains high levels of oxalic acid which binds to iron (II) ions and prevents its absorption in the small intestine. Boiling Water Spinach mitigates this issue by reducing the concentration of oxalic acid thus releasing the bound iron ions.
1.3.2 Iron (II)

The Iron (II) ions in spinach react with oxalic acid and water to produce dihydrate iron (II) oxalate, which binds the iron ions to oxalic acid as shown in figure 2. The equation for this reaction is shown below:
Fe + H2C2O4 + 2H2O → FeC2O4•2H2O + H2
Oxalic acid is a common reducing agent found in most plant-based foods, its conjugate base, oxalate, is a chelating agent for metal ions such as iron (II). A study by Muhammad Shoaib Akhtar had shown that boiling Spinach for 15 minutes reduces the concentration of oxalic acid by 50%, allowing more iron (II) ions to unbind and become free iron ions within the spinach, increasing availability of iron.

Iron (II) is a transition metal with 6 valences electrons in the d-orbital as shown by figure 3. During boiling the iron (II) ions are released from oxalic acid and dissolve into the surrounding water. When iron (II) ions dissolve in water, they form a complex with water called hexaaquairon (II) with the molecular formula [Fe(H2O)6]2+
However, Boiling for extended periods of time breaks down the cellular membranes, allowing the free iron ions to diffuse out of the spinach leaves and into the surrounding water, lowering the nutritional content of the spinach even though more iron ions are being released from oxalic acid.
Iron (II) ions in the spinach extract can be tested by a redox reaction. The iron ions are oxidized by potassium permanganate, while itself is reduced under acidic conditions as shown by equation 3. This involves the transfer of electrons from iron to potassium permanganate. The manganate (VII) ions oxidise iron (II) to iron (III) ions.
These can be represented by the half-equations:
Oxidation Half-Equation 1: Fe2+ ⟶ Fe3+ + e-
Reduction Half-Equation 2: MnO4- + 8H+ +5e- ⟶ Mn2+ + 4H2O
These two equations can be combined to give us the overall equation:
Overall Equation 3: MnO4- + 8H+ + 5 Fe2+ ⟶ Mn2+ + 4H2O + 5Fe3+
1.4 Hypothesis
My hypothesis would be that longer boiling periods will produce lower iron concentrations in water spinach as prolonged exposure to heat degrades the structural integrity of the cell membranes of spinach leaves. As such the cell membrane becomes more permeable to larger molecules and ions such as iron which would leak out of the cells and into the surrounding water. Hence this will result in increasing concentration of Iron (II) ions which will be present which will be oxidized to Iron (III) in the spinach extract.
1.5 Chemicals and apparatus list
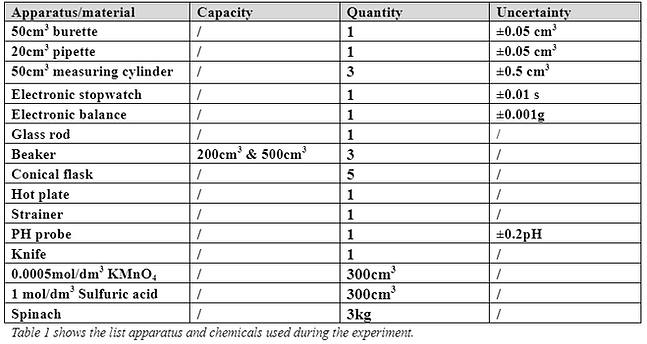
1.6 Table of Variables and Control

2. Methodology
Justification for using spinach extract
The decision was made to use spinach extract to determine the amount of iron leached rather than blending the boiled spinach and determining the amount of iron remaining in the spinach leaves. This is because blended spinach contains several other plant compounds including antioxidants such as kaempferol which would affect the oxidation reaction of iron (II) ions with potassium permanganate, making the iron content appear to be higher than expected. Furthermore, blending spinach would produce a very dark green solution due to the presence of several pigments found in the spinach leaves such as chlorophyll and carotene. This makes the end point of titration harder to identify as the colour change would not be as noticeable. Hence spinach extract was used for titration to circumvent the issues that come with titrating with blended spinach.
2.1 Preliminary Trials
Initially during my preliminary trials, I had considered using Ethylenediaminetetraacetic acid (EDTA) instead of acidified potassium permanganate to chelate the iron (II) ions in the spinach. However upon titrating, I realized that the reaction of EDTA with iron was not as vigorous as I thought, making the end point difficult to identify especially due to the presence of organic compounds and chlorophyll pigments in the water spinach extract. With this in mind I changed the titrant to a stronger oxidizing agent, acidified potassium permanganate.
During preliminary testing with acidified potassium permanganate, I wanted to identify to ideal concentration to be used for titration such that the reaction did not occur too quickly nor too slowly. Reviewing literature values, I came to the realization that because of low concentrations of iron in Spinach and vegetables, I would have had to settle with a slightly higher concentration of KMnO4 otherwise the uncertainty whilst measuring the KMnO4 powder would have been too great.
Furthermore, during titration, I had noticed that the oxidized water spinach extract would be pink, indicating its endpoint, for a short period of time before slowly changing back to its original green colour. Upon further research, I had realized that this was due to the presence of organic compounds in the water spinach extract reacting slowly with the KMnO4. However this reaction is much slower than the redox reaction with Iron (II) thus I managed to adjust my procedure accordingly such that the first hint of the pink colour “lingering” in the water spinach extract would indicate that all of the iron (II) ions in the water spinach extract have been oxidized to iron (III) ions. Thus through these efforts of troubleshooting my experiment and methodology, I was able to begin data collected soon after.
The following method below shows the final procedure used after reflecting on the preliminary trials
2.2 Procedures
Preparation of KMnO4 solution

0.2g of KMnO4 powder was measured using electronic weighing scale. 250cm3 of distilled water was then measured out using a 100cm3 measuring cylinder. The potassium permanganate powder was then added to the distilled water to obtain 250cm3 of 0.005mol dm3 of KMnO4 solution. Half of the KMnO4 solution was poured away and replaced by the same volume of water to dilute the concentration of the solution. This dilution process was repeated until a 0.0005mol dm3 of KMnO4 solution was obtained. This solution was added to a 250cm3 volumetric flask.
Obtaining spinach extract solution
100g of spinach inclusive of the step was weighed on an electronic weighing scale. The spinach was cut into small pieces using a knife and a cutting board to increase its surface area to volume ratio, maximizing the amount of iron that will be released into the water. 500cm3 of water was measured out using a 1L measuring cylinder and poured into a 1L beaker. The water was then heated until boiling using a hot plate. Upon boiling, 100g of spinach was added and a stopwatch was started. The surface area of the spinach was assumed to be the same as their masses are the same, hence the difference in iron(II) ions released due to inconsistencies in surface area would be negligible. After 10 minutes had elapsed, a metal strainer was used to filter out the spinach from the spinach extract solution. The extract was left to cool in a refrigerator for 15 minutes before titrating for safety reasons. The entire procedure was repeated for each boiling period.

Titration of spinach extract with KMnO4 solution
25cm3 of spinach extract was measured out using a measuring cylinder and poured into a conical flask, following which 25cm3 of 1mol/dm3 sulfuric acid was measuring with a measuring cylinder and added into the same conical flask. 0.0005mol/dm3 KMnO4 was titrated against this until a bright pink colour was obtained, indicating that all the Fe2+ had been oxidized to Fe3+. The titration results were recorded onto a table. After each titration, the apparatus were rinsed with distilled water before commencing with the next titration. The entire process was repeated for the various iron extracts of different boiling times
3.1 Qualitative observations

Initially, the Spinach Extract is green in colour due to the presence of iron (II) ions and pigments such as chlorophyll in the water. As evident from figures 6 to 8, longer boiling periods yielded darker green colours, signifying a higher concentration of Iron (II) ions present. A higher concentration of pigments from the leaves such as chlorophyll and carotene also contribute to the darkening of the spinach extract.
Upon titration with the KMnO4 solution, the KMnO4 solution is rapidly reduced, and changes colour from dark purple to colourless. A light pink solution is obtained, signifying that all iron (II) ions in the solution have oxidised to iron (III).The light pink colour gradually fades away and the solution returns to its original green colour
3.2 Raw data

4. Data processing
4.1 Table of formulae

4.2 Processed data

5. Discussion
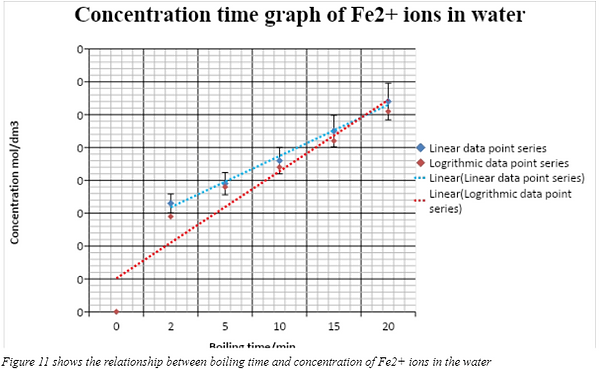
With reference to figure 7 there is an apparent strong linear relationship between the 5 data points (2, 5, 10, 15,20minutes) for iron content in the water, thus showing that as boiling time increases, more iron (II) ions diffuse out of the spinach. Cross referencing to the qualitative data, longer boiling periods had higher concentrations of iron (II) ions present in the water spinach extract, this caused it to be darker green in colour due to the presence of more Iron (II) ions. Longer boiling periods showed a slightly more vigorous reaction as the change in colour was quicker as compared to shorter boiling times. This can be attributed to the higher concentration of iron (II) ions thus increasing the rate of reaction.
At first sight, the graph seems to corroborate with the hypothesis, with a very high R2 value of 0.9922 to showing that a possible linear trend line exists. However due to the limited number of data points, it would be difficult to tell whether this trend continues for longer boiling times. According to an evidence based article by Franziska spritzler, raw spinach contains approximately 3.5mg of iron. Hence it can be hypothesised that with extended periods of boiling, ie 30,40,50,60 minutes, the graph would plateau as no more iron is left in the spinach.
Furthermore referencing again to figure 7 when the data point of 0 boiling time was included, a logarithmic trend line may be observed, giving an R2 value of 0.9833. This serves to show that in the initial first few minutes of boiling, a large portion of the iron is already released. This alternative trend line also corroborates with the hypothesis and prediction that the graph would plateau for longer boiling times, hence is a more plausible trend line and better explains the loss of iron in spinach overtime. Cross referencing to table 5, it can be seen that after just 2 minutes of boiling, more than half, approximately 51.9 % of the iron had already been released. The remainder 48.1% took another 18 minutes before it was all released into the surrounding water.
A possible explanation for this is that prior to boiling, a portion of the iron (II) ions was not bound to oxalic acid. Thus the short boiling time broke down the cell membrane and cell wall of spinach cells, releasing the unbound iron (II) ions found in the spinach into the surrounding water thus explaining the sudden jump in the iron content of the water spinach extract between 0 and 2 minutes of boiling. Extended boiling periods would lead to the iron ions bound to oxalic acid being gradually released and diffuse into the surrounding water. Hence, the linear trend line in figure 7 actually refers to the rate at which iron ions bound to oxalic acid are released and diffused into the surrounding water.
The logarithmic trend line in figure 7 shows that boiling causes a dramatic loss in iron content in the water spinach. The water spinach would have lost approximately 73.4% or 2.569 mg of iron by the 10th minute boiling.
Data analysis
The raw data shows that after 20 minutes of boiling, 3.57 mg of iron was present in the water spinach extract. This value of 3.57 is also in line with the literature values obtained from the United States Department of Agriculture, which state that cooked spinach has an average amount of 3.5mg of iron, thus supporting the data collected.
Upon extension of boiling time to 30 minutes, there was negligible change in the mass of iron present in the water, thus suggesting that most if not all of the iron (II) ions in the water spinach had been released from oxalic acid and into the water.
The experimental data obtained was slightly higher than the values recorded by Namita Bhardwa which employed the use of a spectrophotometer to measure the absorbance of iron ions in the dry mass of water spinach with respect to various boiling times. The slight deviation in data could possibly be attributed to the presence of organic compounds in the water spinach extract such as oxalic acid as mentioned earlier. Oxalic acid reacts much slower with the KMnO4 solution relative to the iron ions, thus in the time it took for the Fe2+ ions to oxidize, it is possible that 0.07mg of iron had reacted with the oxalic acid present. Permanganate ion removes electrons from oxalic acid molecules since they are more electronegative and thereby oxidizes the oxalic acid.
A possible side reaction between Oxalic acid and acidified potassium permanganate can be represented by the following equation:
Reduction half reaction: 2KMnO4 + 3H2SO4 → K2SO4 + 2MnSO4 + 3H2O + 5[O]
Oxidation half reaction: 5 H2C2O4 + 5[O] → 5H2O + 10CO2
Overall reaction: 2KMnO4 + 3H2SO4 + 5 H2C2O4→ K2SO4 + 2MnSO4 + 8H2O + 10CO2
Furthermore, the calculated experimental error of 2.11% was found to be lesser than the percentage uncertainty of 14.2%, thus this implies the results are accurate and the experimental data obtained falls within the accepted limits
6. Evaluation
6.1 limitations
One limitation of this investigation would be the lack of standardisation of KMnO4. Since this was not carried out, the exact concentration of KMnO4 was unknown, leading to inaccuracy during titration as the concentration of the KMnO4 could be higher or lower than expected, leading to inaccurate measurement on the concentration of iron leached from the spinach leaves and into the surrounding water. Thus an extension for this investigation, standardised KMnO4 will be used to ensure more reliable data collection.
Another noticeable limitation would be the presence of organic compounds such as oxalic acid which slowly reacts with Potassium permanganate. This not only makes the end point of titration more uncertain, but also increases the volume of KMnO4 reacted, thus leading to a mass of iron lost to be greater than expected as stated earlier. Due to the short amount of time it takes to titrate KMnO4 with the spinach extract, the deviation from literature values was approximated to be 0.07 mg. A research paper by Aldo Wenedy on the effects of various cooking methods on the composition of spinach showed that a way around this issue would be to boil the dry mass of the spinach through multiples cycles of heating in an oven.
Lastly, the limited range of data values is also a limitation in this experiment as stated prior given the time constraints. This makes it difficult for an accurate trend line to be obtained since there are large gaps between each data value. A prime example of this would be the contrast between the two curves in figure 7 due to the addition of a single data point. Thus the trend line obtained for this investigation only serves as a general trend line for short boiling periods since data points were not collected for extended periods of boiling. Given more time, more replicates can be obtained for boiling times with 1 minute increments compared to 5 and effects of extended boiling periods may also be tested.
6.2 Strengths
One strength of this investigation would be the wide spread applicability of the methodology. The methodology can be used to determine the iron content in various other vegetables and fruits and is not limited to just spinach. This allows us to determine the effect of boiling over a wide variety of foods and determine the nutritional benefits of boiling vegetables with other cooking methods such as steaming or microwaving by comparing the amount of iron left within each food.
Furthermore, the methodology applied though simple is still reliable and accurate, allowing ease with the data collection. This investigation employs use of very simple apparatus and chemicals hence it can be easily understood and followed. Furthermore, the simple set up also means that more people are also able to replicate this experiment for different types of vegetables or perhaps different species of spinach to determine which has the highest iron content.
6.3 Conclusion
From the data collected and graphs drawn, there is an obvious negative correlation between the boiling time and concentration of iron, leading to a larger amount of iron being oxidized by potassium permanganate. Longer boiling periods yielded increasing amounts of iron in the water which is represented by both a logarithmic trend line, hence showing that Water Spinach losses a significant amount of its nutritional value in the first few minutes of boiling. Thus, in order to maximize the nutritional value of spinach, a shorter boiling time is ideal as it releases the iron from oxalic acid while still allowing the spinach to retain its iron content.
Bibliography
-
Akhtar, M. S., Israr, B., Bhatty, N., & Ali, A. (2011). Effect of Cooking on Soluble and Insoluble Oxalate Contents in Selected Pakistani Vegetables and Beans. International Journal of Food Properties, 14(1), 241–249. doi: 10.1080/10942910903326056
-
bardwaj, N. (2015, July 1). Spectrophotometric Analysis of Iron Content in Spinach . Retrieved from http://www.cvruresearch.org/PDF/11.pdf.
-
Beck, L., & Beck, L. (2018, April 30). Is spinach more nutritious raw or cooked? Retrieved November 16, 2019, from https://www.theglobeandmail.com/life/health-and-fitness/ask-a-health-expert/is-spinach-more-nutritious-raw-or-cooked/article565617/.
-
Bonsmann, S. S. G., Walczyk, T., Renggli, S., & Hurrell, R. F. (2007). Oxalic acid does not influence nonhaem iron absorption in humans: a comparison of kale and spinach meals. European Journal of Clinical Nutrition, 62(3), 336–341. doi: 10.1038/sj.ejcn.1602721
-
Gunnars, K. (2019, May 14). Spinach 101: Nutrition Facts and Health Benefits. Retrieved January 24, 2020, from https://www.healthline.com/nutrition/foods/spinach#plant-compounds
-
Howland, G. (2019, May 24). Natural Remedies for Anemia. Retrieved November 15, 2019, from https://www.mamanatural.com/anemia-during-pregnancy/.
-
Iron(II) oxalate. (2019, October 26). Retrieved November 16, 2019, from https://en.wikipedia.org/wiki/Iron(II)_oxalate.
-
“Iron.” Iron, https://www.chemguide.co.uk/inorganic/transition/iron.html.
-
Swanson, D. (2019). Targeting children to beat anaemia . Retrieved from http://www.thenewhumanitarian.org/report.aspx?reportld=85882.
-
Shimada, Y. (2014). The Eeffect of Soaking on the Soluble Oxalic Acid Content of Spinach. The Eeffect of Soaking on the Soluble Oxalic Acid Content of Spinach, 13. Retrieved from https://pdfs.semanticscholar.org/330c/ee555b6ccef1a3e10bba876bbe801924c3a2.pdf
-
spritzler, F. (2018, July 18). 11 Healthy Foods That Are Very High in Iron. Retrieved October 29, 2019, from https://www.healthline.com/nutrition/11-healthy-iron-rich-foods.
-
Styles, S. (2018, December 2). How to Keep Iron Nutrients in Spinach When Cooking. Retrieved January 19, 2020, from https://healthyeating.sfgate.com/keep-iron-nutrients-spinach-cooking-9374.html
-
Why Most Iron In Spinach Is Useless. (n.d.). Retrieved November 16, 2019, from https://www.nutritics.com/p/news_Why-Most-Iron-In-Spinach-Is-Useless.
-
Water Treatment Solutions. (n.d.). Retrieved November 2, 2019, from https://www.lenntech.com/periodic/elements/fe.htm.



